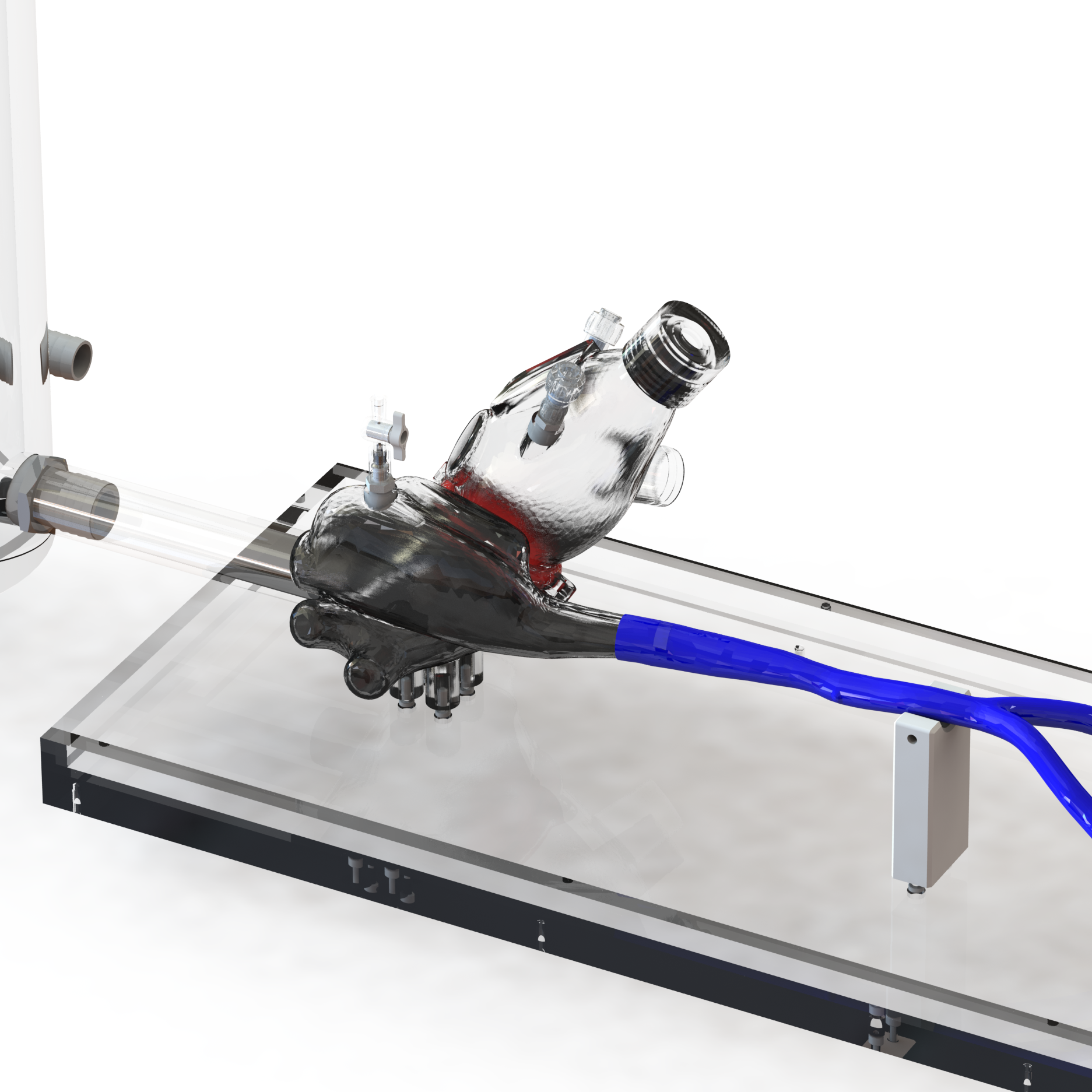
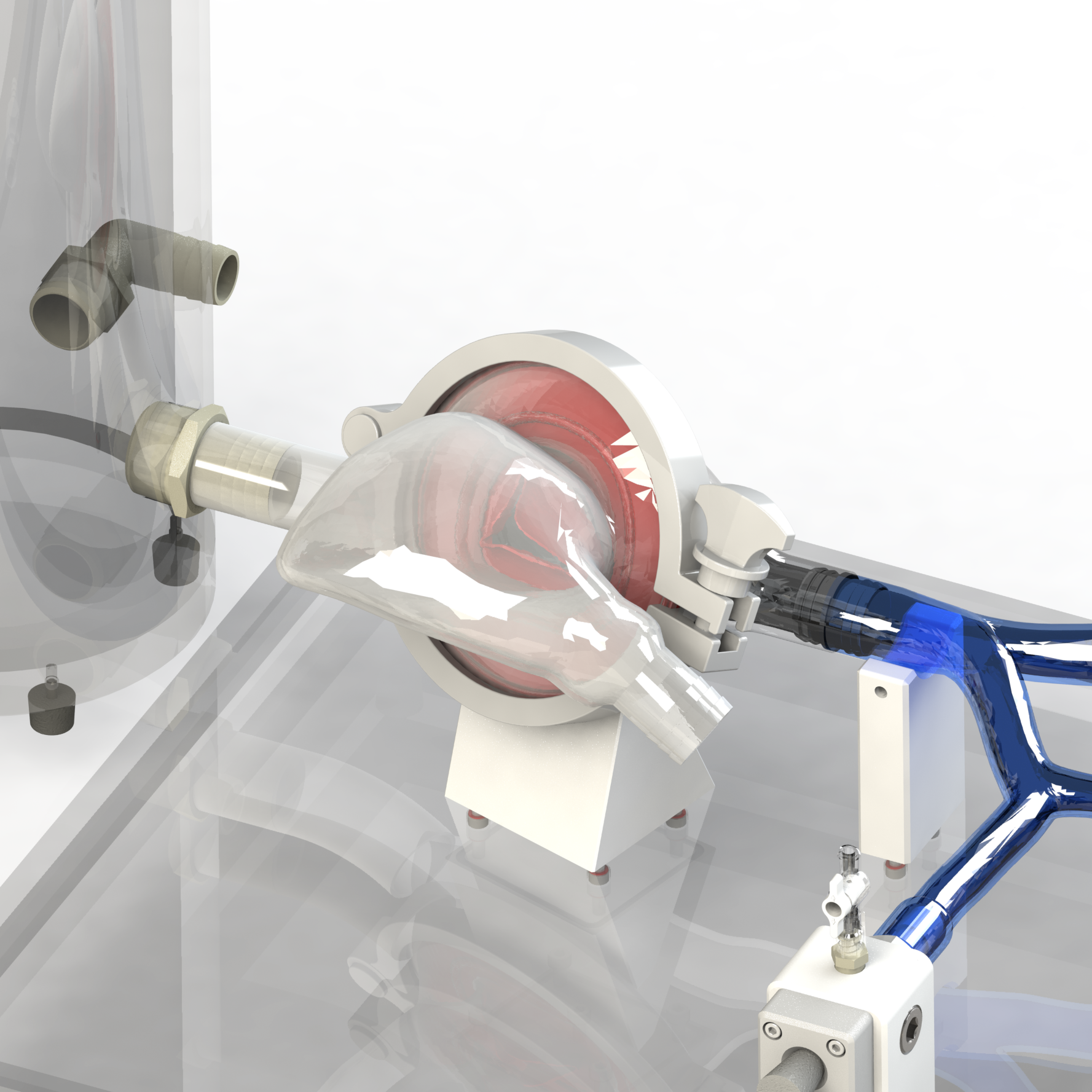
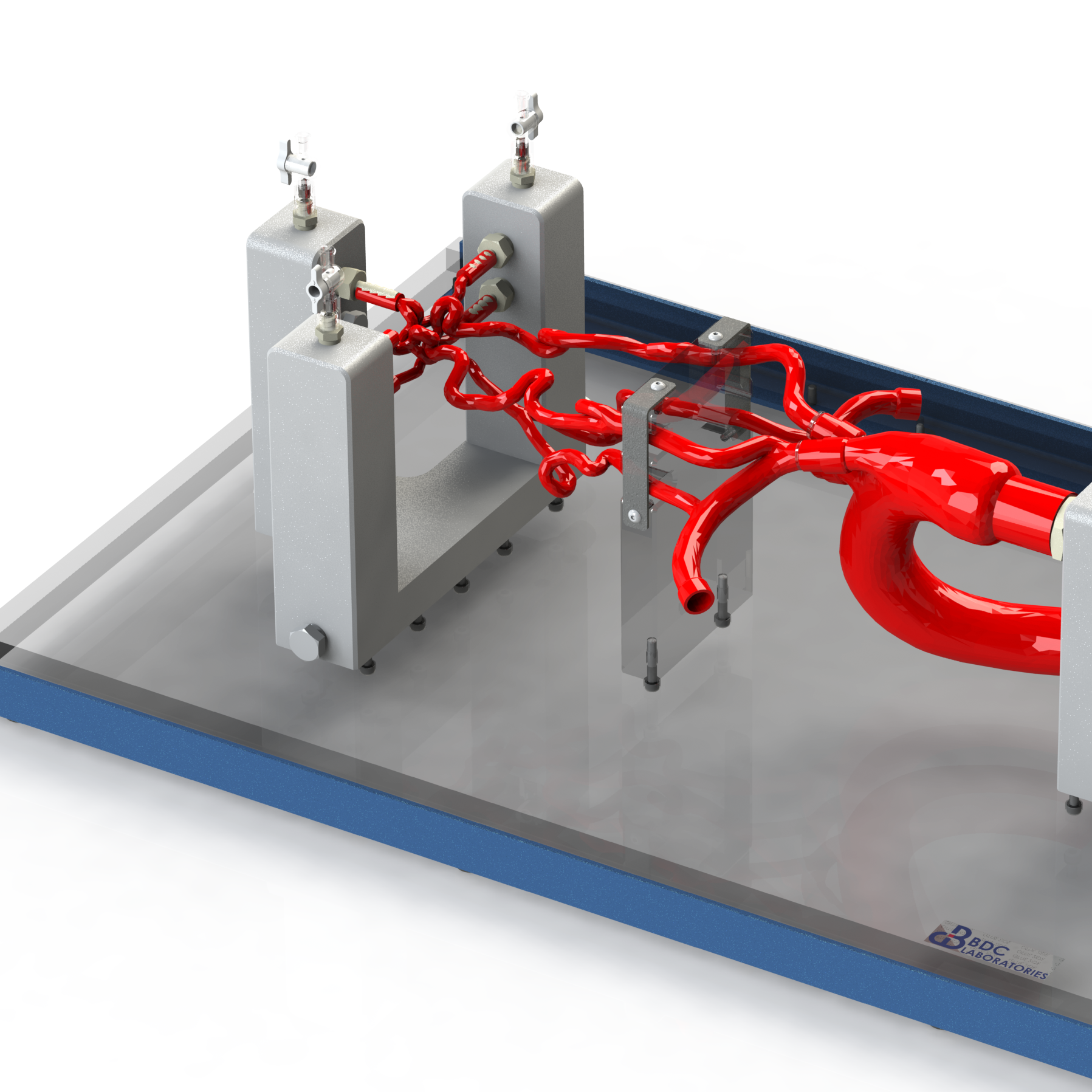
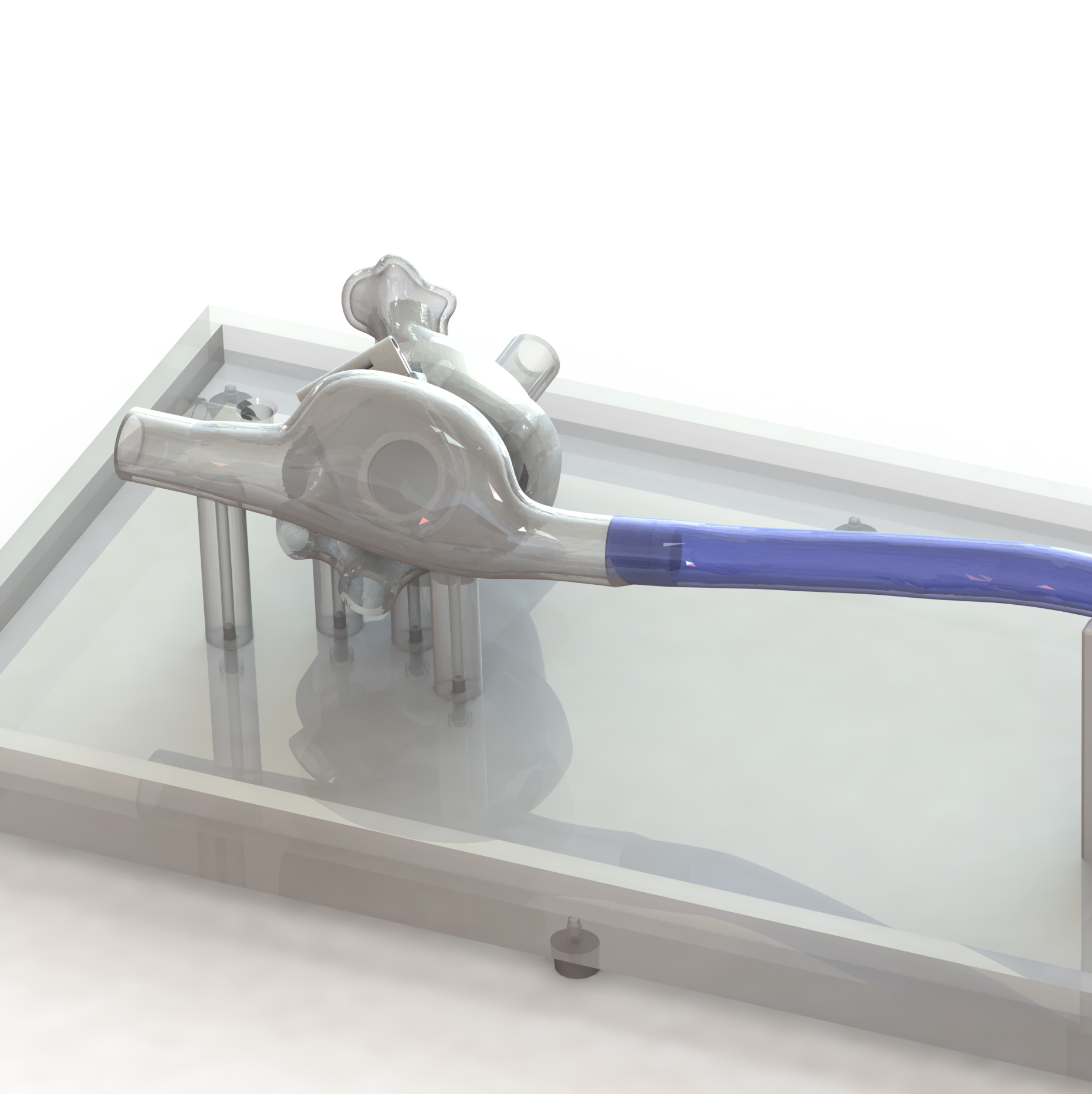
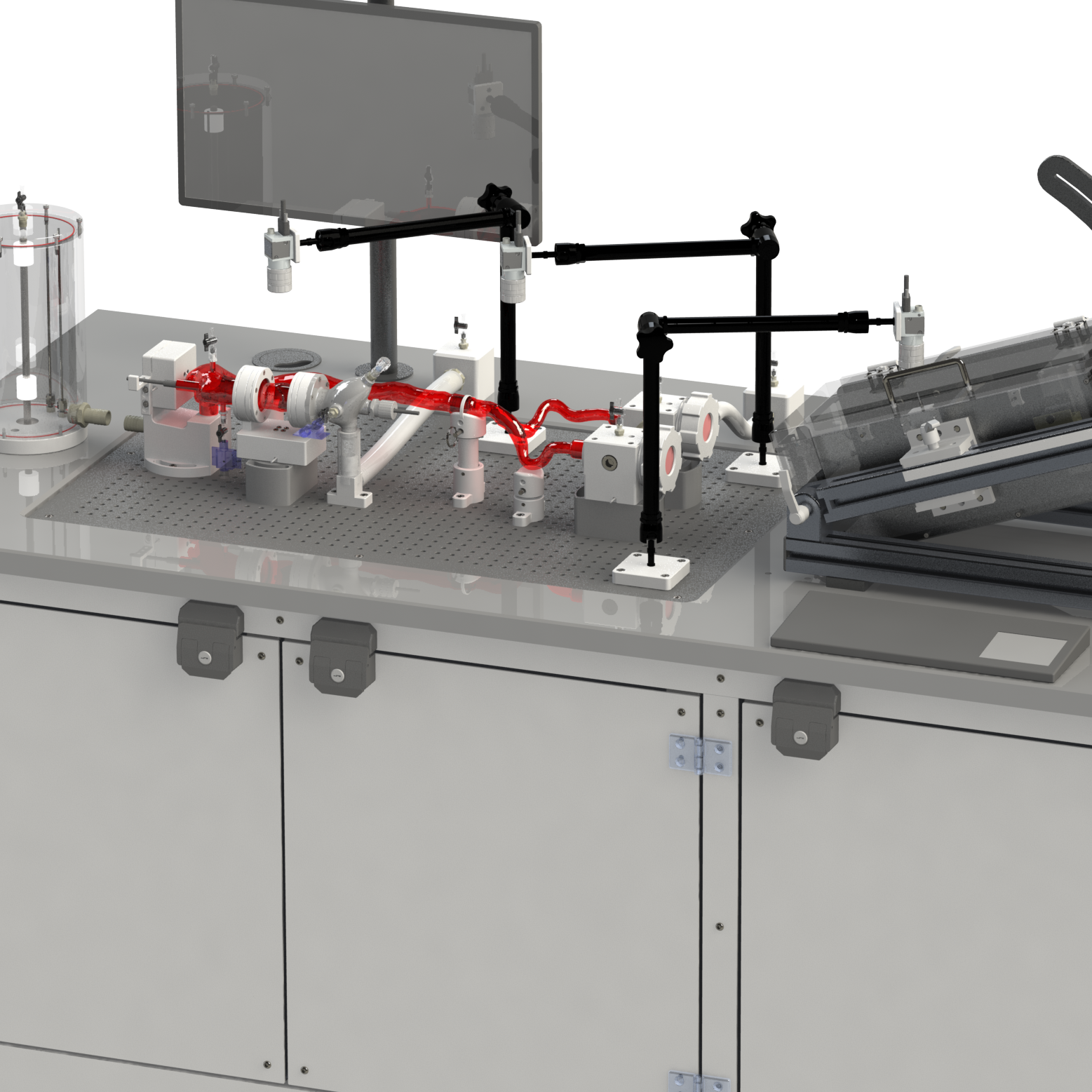
A simulated use system, or mock circulatory loop, that has been tailored for the testing and evaluation of cardiovascular medical devices becomes a simulation solution. Simulation solution systems seek to mimic the pressure, flow and temperature conditions of the human cardiovascular system to facilitate study of critical endovascular and heart valve device characteristics and design parameters in a predictable, controlled, and reproducible manner. The simulated use test system further presents an apparatus where specific performance attributes of a technology can be quantified and qualified during design verification testing, in addition to providing a focused demonstration, marketing and physician training tool.
BDC Laboratories designs and builds standard, as well as custom simulated use systems that include clinically relevant silicone vascular pathways to aid in the evaluation of pulsatile flow device interactions, endovascular and transcatheter heart valve implant positioning, implant deployment accuracy, implant sizing, conformability of a stent graft or stent to vessel wall, delivery system trackability, pushability, torquability, deployment force, and TAVR valve-in-valve deployments.
Move Forward with BDC
Using a mix of their tried-and-true systems and new electromechanical components, BDC produced test equipment that precisely met our needs. In addition to being on scope and schedule, I was impressed by the thought that Ben McCloskey put into the GUI and overall usability of the system. He and Craig were a pleasure to work with.
The Foundry
Read More