RDTL-0200 Radial Durability System
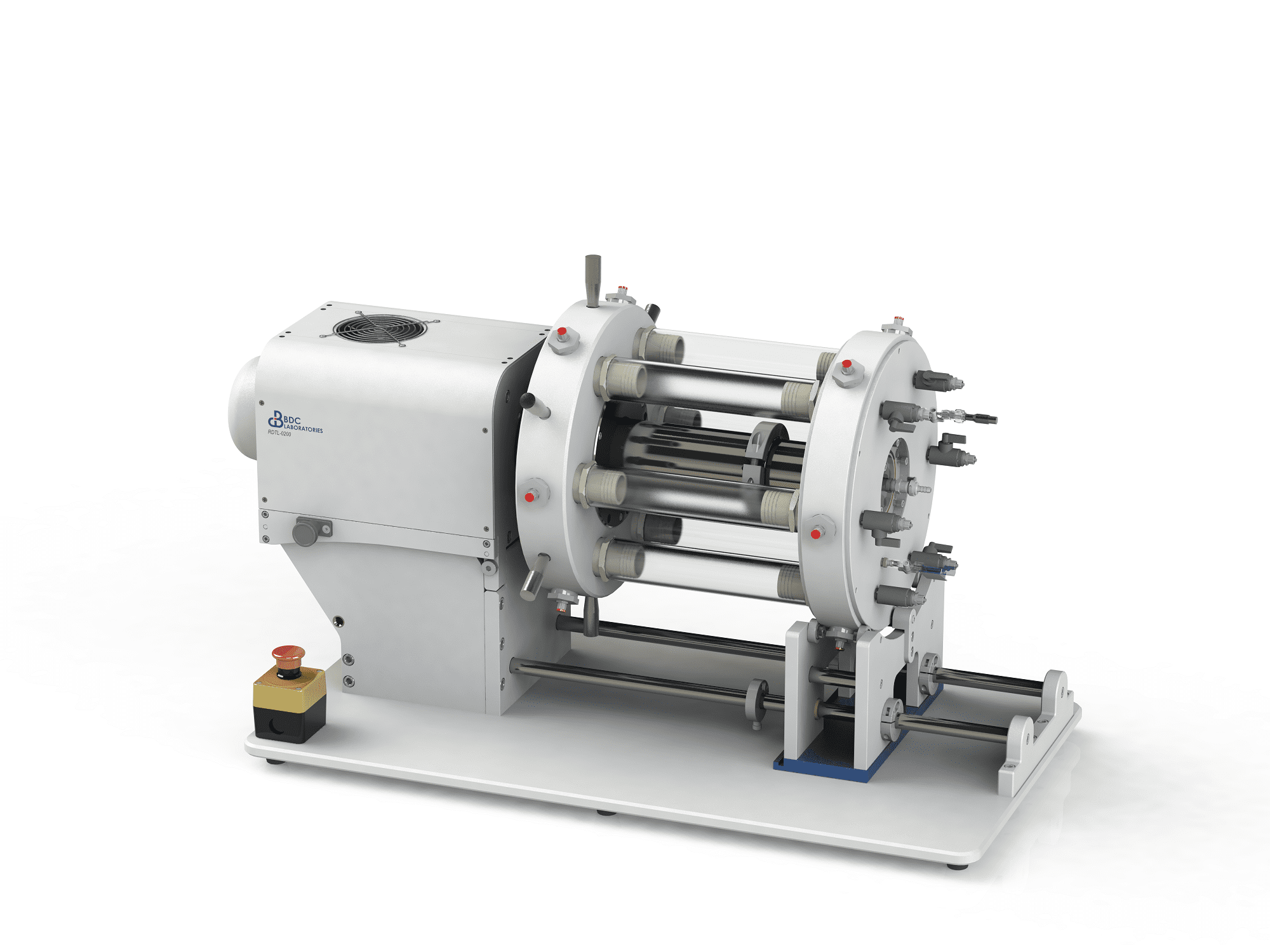
BDC Laboratories’ RDTL-0200 radial durability test system for endovascular devices (e.g. stents, stent grafts, transcatheter heart valve frames, left atrial appendage (LAA) occluders) incorporates our internally developed and patented RB-5™ Drive System and Dual-Drive Emulator technology. The combination of these two technologies produces concurrent pressurization at either end of the test sample with every cycle, eliminating the need for two drivers and thus, yielding increased test system reliability.
The RDTL-0200 radial durability test system is controlled and monitored through BDC’s Statys® RDT closed-loop control and monitoring software. BDC’s pulsatile durability tester achieves the requirements of many different endovascular technologies, under a vast array of configurations, while meeting the international and US test standard requirements of ISO 25539 and ASTM F2477, respectively.